Equilibrium
Conditions That Affect Reaction Rates
- Reactions speed up when temperature is increased
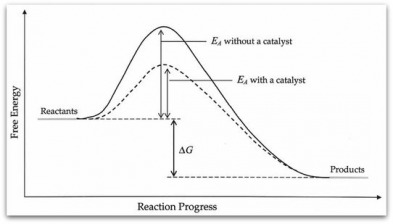
- Activation energy (Ea)- a minimum energy needed for a reaction to occur
- Energy in a collision > Ea à will result in a reaction
- Energy in a collision < Ea à molecules will bounce apart unchanged
- Speeds increase with temperature
- Catalyst- a substance that speeds up a reaction without being consumed
- Enzymes = catalysts is on our bodies
- Provides new pathway for reaction
- When “species” that appear on both sides of the arrow are canceled, the end result is the reaction
- Cl + O3 + O +ClO à ClO + O2 + Cl + O2 becomes O + O3 à 2O2
One chlorine atom can catalyze the destruction of about one million ozone molecules per second
- Cl + O3 + O +ClO à ClO + O2 + Cl + O2 becomes O + O3 à 2O2
Higher Temperatures à Higher Speeds à More high-energy collisions à More collisions that break bonds à Faster reaction
Recall (13.8): average kinetic energy of a group of molecules is directly proportional to the temperature (k).
Ozone fact: Ozone atoms are too reactive to exist near the earth's surface , they do exist in the upper atmosphere.
Ozone fact: Ozone atoms are too reactive to exist near the earth's surface , they do exist in the upper atmosphere.