Equilibrium
The Equilibrium Constant: An Introduction
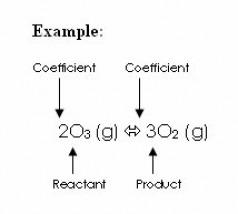
Law of chemical equilibrium- a general description of the equilibrium condition.
aA + bB <--> cC + dD
A,B,C,D=chemical species
a,b,c,d=coefficients in balanced equation
Equilibrium expression: K= [C]c [D]d
[A]a[B]b
K is the constant called the equilibrium constant.
Brackets are the concentrations of chemical at equilibrium (in units of mol/L)
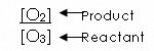
To obtain the equilibrium expression, we place the concentration of the product in the numerator and the concentration of the reactant in the denominator.
-Subscript zeros next to square brackets are used to indicate initial concentrations.
-Equilibrium concentrations are not always the same.
-The equilibrium constant, which depends on the ratio of the concentrations, remains the same.
-Each set off equilibrium concentrations is called an equilibrium position.
-The law of chemical equilibrium predicts that the value of K should be the same for both experiments.
-Equilibrium concentrations are not always the same.
-The equilibrium constant, which depends on the ratio of the concentrations, remains the same.
-Each set off equilibrium concentrations is called an equilibrium position.
-The law of chemical equilibrium predicts that the value of K should be the same for both experiments.