Equilibrium
The Equilibrium Condition
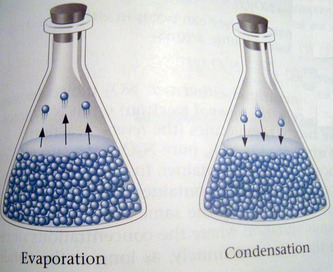
Equilibrium- the exact balancing of two processes, one of which is the opposite of the other.
The rate of the two reactions has to be the same.
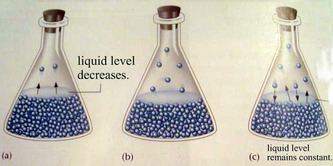
The equilibrium state occurs when the rate of evaporation exactly equals the rate of condensation.
Many chemical reactions “stop” far short of completion when they are allowed to take place in a closed container.
An example is the reaction of nitrogen dioxide to form dinitrogen tetroxide.
NO2(g) + NO2(g)à N2O4(g)
Reddish-brown Colorless
Chemical equilibrium- a dynamic state where the concentrations of all reactants and products remain contanst.
An example is the experiment:
-Pure NO2 is placed in an empty, sealed glass vessel at 25°C. The initial dark brown color will decrease in intensity as the NO2 is converted to colorless N2O4. The intensity of the brown color eventually becomes constant, which means that the concentration of NO2 is no longer changing. This observation is a clear indication that the reaction has “stopped” short of completion.
Equilibrium is reached whether pure NO2, pure N2O4, or a mixture of NO2 and N2O4 is initially placed in a closed container.
The rate of the two reactions has to be the same.
The equilibrium state occurs when the rate of evaporation exactly equals the rate of condensation.
Many chemical reactions “stop” far short of completion when they are allowed to take place in a closed container.
An example is the reaction of nitrogen dioxide to form dinitrogen tetroxide.
NO2(g) + NO2(g)à N2O4(g)
Reddish-brown Colorless
Chemical equilibrium- a dynamic state where the concentrations of all reactants and products remain contanst.
An example is the experiment:
-Pure NO2 is placed in an empty, sealed glass vessel at 25°C. The initial dark brown color will decrease in intensity as the NO2 is converted to colorless N2O4. The intensity of the brown color eventually becomes constant, which means that the concentration of NO2 is no longer changing. This observation is a clear indication that the reaction has “stopped” short of completion.
Equilibrium is reached whether pure NO2, pure N2O4, or a mixture of NO2 and N2O4 is initially placed in a closed container.