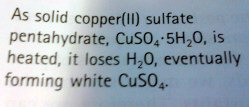Equilibrium
Heterogeneous Equilibria
Homogeneous Equilibria- all substances are in the same state
Heterogenous Equilibria- substances that involve more than one state
Acid-base reactions are also examples of heterogeneous equilibria as they involve both pure liquids (the solvent) and the dissolved species.
Heterogenous Equilibria- substances that involve more than one state
Acid-base reactions are also examples of heterogeneous equilibria as they involve both pure liquids (the solvent) and the dissolved species.
- The position of a heterogeneous equilibrium does not depends on the amounts of pure solids or liquids present.
- The concentrations of pure solids or pure liquids involved in a chemical reaction are not included in the equilibrium expression for the reaction. Applies only to solids or liquids.
- The concentrations of pure solids or pure liquids involved in a chemical reaction are not included in the equilibrium expression for the reaction. Applies only to solids or liquids.